New Materials
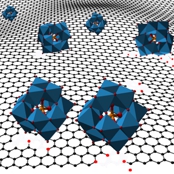 Hybrid materials. Polyoxometalates are nanometer-sized electroactive inorganic clusters alike oxides quantum dots. Anchoring them in carbons or conducting polymers allows for the harnessing of their electrochemical (faradaic) properties as electrodes for energy storage.
Hybrid materials. Polyoxometalates are nanometer-sized electroactive inorganic clusters alike oxides quantum dots. Anchoring them in carbons or conducting polymers allows for the harnessing of their electrochemical (faradaic) properties as electrodes for energy storage.
H ybrid Nanostructures.
ybrid Nanostructures. 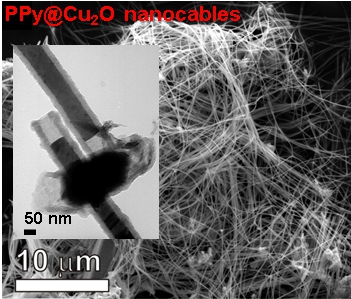
 Conducting polymers and metals can be made to grow together to form self-assembled nanostructures: nanowires, nanocables or nanosnakes are some of the most spectacular. It also works with oxides.
Conducting polymers and metals can be made to grow together to form self-assembled nanostructures: nanowires, nanocables or nanosnakes are some of the most spectacular. It also works with oxides.
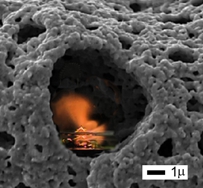 Fractal Porosity. Fractal granularity.
Fractal Porosity. Fractal granularity.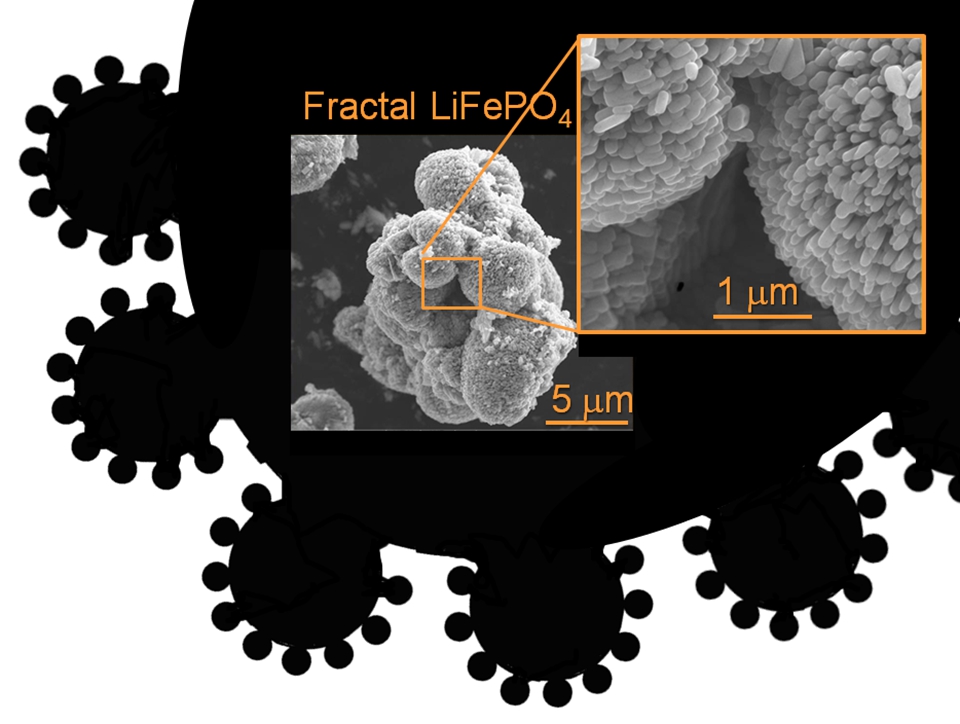 How to induced porosity in a material spanning macro meso and micro-pores? Nickel was a beautiful example of fractal microstructure engineering.Then we made LiFePO4 active electrode materials with fractal granularity.
How to induced porosity in a material spanning macro meso and micro-pores? Nickel was a beautiful example of fractal microstructure engineering.Then we made LiFePO4 active electrode materials with fractal granularity.
 Matrix Chemistry. Silver Nanoparticles and Nanostructures in Biopolymers. A polymer matrix is an elegant way to control the growth of nanoparticles. The reduction of Ag+ ions inside agar-agar gels led to protected Ag nanoparticles and nanostructures
Matrix Chemistry. Silver Nanoparticles and Nanostructures in Biopolymers. A polymer matrix is an elegant way to control the growth of nanoparticles. The reduction of Ag+ ions inside agar-agar gels led to protected Ag nanoparticles and nanostructures
New Chemical Compounds
Most of the time we seek to prepare materials and study their properties and performance. But sometimes we just synthesize new chemical compounds with no application in mind
Wh en they were discovered (or invented?) these compounds were not even advanced materials. Ag2Cu2O3 was the first mixed oxide of copper and silver and it was synthesized because it didn’t exist. Not even in nature, as far as we know.
en they were discovered (or invented?) these compounds were not even advanced materials. Ag2Cu2O3 was the first mixed oxide of copper and silver and it was synthesized because it didn’t exist. Not even in nature, as far as we know.
Ag2Cu2O3 : The First Silver-Copper Oxide. P. Gómez-Romero*, E. Tejada-Rosales, M.R. Palacín. Angew.Chem. 1999, 111(4), 544-6. Angew.Chem.Int.Ed.Engl. 1999, 38(4), 524-5.
Latter on, this new compound was patented as an efficient catalyst for the partial oxidation of methanol to formaldehide and as a electrode for primaryLi batteries
Thermally stable catalysts comprising copper-silver mixed oxides, useful for oxidation of organic substrates, e.g. for the partial oxidation of alcohols. Patent Number(s): WO2003103831-A; WO2003103831-A1; ES2197013-A1; AU2003232853-A1; ES2197013-B1; AU2003232853-A8 Inventor(s): GOMEZ-ROMERO P, TEJADA-ROSALES E, MUNOZ-ROJAS D, CASAN-PASTOR N, MESTL G, WOELK H, CSIC and MAX PLANCK INST(PLAC-C), 30 05 2003. Derwent Primary Accession Number: 2004-071300 [48]